醫(yī)療器械注冊(cè)要多少錢���?醫(yī)療器械注冊(cè)要多久??是企業(yè)普遍關(guān)心的兩個(gè)問(wèn)題��。近日�����,藥監(jiān)總局發(fā)布2022年2月審結(jié)轉(zhuǎn)出注冊(cè)項(xiàng)目審評(píng)用時(shí)情況一覽表�,一起來(lái)看一下���。
醫(yī)療器械注冊(cè)要多少錢���?醫(yī)療器械注冊(cè)要多久?是企業(yè)普遍關(guān)心的兩個(gè)問(wèn)題����。近日,藥監(jiān)總局發(fā)布2022年2月審結(jié)轉(zhuǎn)出注冊(cè)項(xiàng)目審評(píng)用時(shí)情況一覽表�����,一起來(lái)看一下。
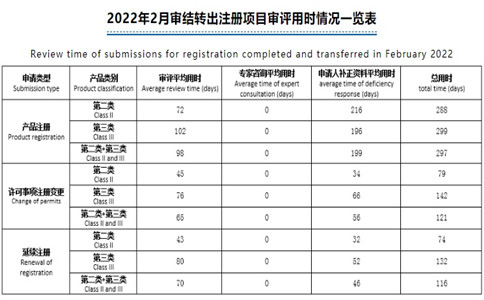
備注:
1.本表涉及時(shí)間均為工作日�。
The time in this table is working days.
2.補(bǔ)正資料平均用時(shí)=補(bǔ)正資料總用時(shí)/相關(guān)項(xiàng)目審結(jié)轉(zhuǎn)出總數(shù)量
average time of deficiency response = total time of deficiency response /total number of completed and transferred submissions with deficiencies.
3.總用時(shí)=審評(píng)平均用時(shí)+專家咨詢平均用時(shí)+補(bǔ)正資料平均用時(shí)
Total time = average review time+ average time of expert consultation + average time of deficiency response.
4.第二和第三類產(chǎn)品平均用時(shí)=第二類和第三類產(chǎn)品用時(shí)/第二類和第三類產(chǎn)品總數(shù)量
Average time for Class II and III products = total treview time of class II and III products/the total number of class II and III product submissions.
5.應(yīng)急審評(píng)用時(shí)不在統(tǒng)計(jì)范圍內(nèi)
Time of emergency review is out of the scope of this statistics.